New Method for Skeletal Rearrangement Enhances the Organic Chemist's Toolbox
In organic synthesis, especially in total synthesis, the basic approach is quite straightforward: commercially available starting materials are expanded primarily through the formation of C-C, C-O, and C-N bonds to form complex molecules. This overarching principle is guided primarily by retrosynthetic analysis and total synthesis ideas such as E.J. Corey’s synthon concept.
But wouldn’t it be exciting to actually break C-C bonds to create new molecules? Can skeletal rearrangement be a powerful addition to drug discovery? Recently, I was able to contribute to such a story when Billy Ng’s research found a way to do a skeletal rearrangement of bilobalide.(JACS Au 2024, 4, 9, 3537–3546) Despite the beauty of this reaction, this rearrangement is quite substrate dependent and a more generalized approach is needed. Scrolling through recent publications, it is clear that most of this work is driven by photochemical reactions. It is therefore not surprising that the latest addition to the skeleton rearrangement toolbox from Richmond Sarpong’s research group is also based on UV irradiation. In their paper published in Science, the researchers from Berkeley University (USA) describe a 1,2-acyl transposition by skeletal rearrangement.
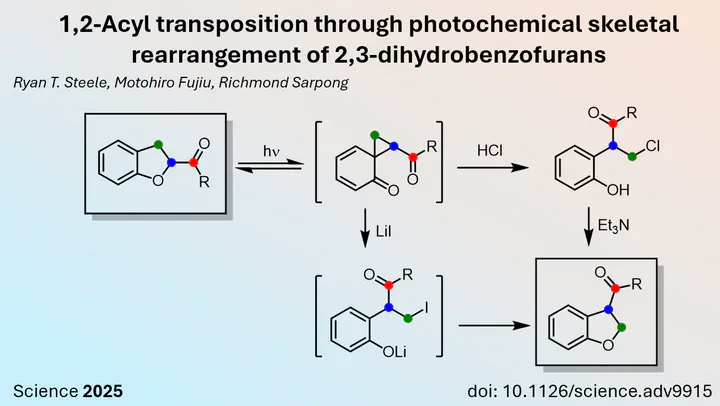
Although 1,3-acyl transpositions are well known for unsaturated systems, their 1,2 counterparts are more difficult to achieve. Again, a well-defined substrate is needed to facilitate this transformation. The Sarpong group decided to use a 2,3-dihydrobenzofuran starting material, which can form a reactive cyclopropane intermediate upon UV irradiation. Addition of halogen, either by acidic conditions such as HCl or by the addition of LiI, could then trigger the opening of the cyclopropane, which was then attacked by the nucleophilic phenol, yielding the 1,2-acyl transferred product.
Although the substrate range is narrow, it must be emphasized that such transformations are far from being easily accessible. Therefore, we can look forward to the coming years to see how such concepts can overcome the limitations of previous synthetic strategies by adding new methods to the organic chemist’s toolbox.
Read more: 1,2-Acyl transposition through photochemical skeletal rearrangement of 2,3-dihydrobenzofurans Science doi:10.1126/science.adv9915