Novel Synthetic Approach for the Synthesis of Pyrroloiminoquinones
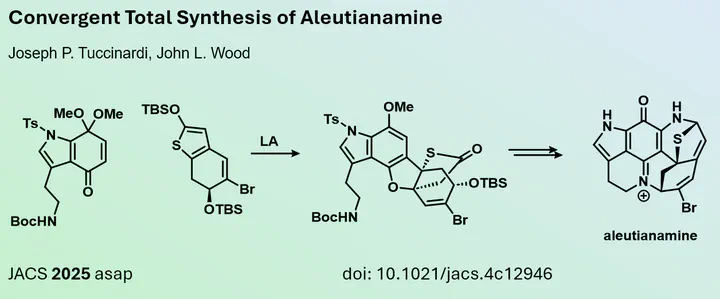
Pyrroloiminoquinones are complex alkaloids that have fascinated generations of synthetic chemists since the 1990s. The isolation of a novel member of this class, aleutianamine, in 2019 triggerd renewed interest within the total synthesis community, resulting in elegant syntheses by Stoltz and Tokuyama in 2023. This novel alkaloid features an unusual N,S-acetal and demonstrates significant biological activity against cancer cell lines at submicromolar concentrations.
In a recent publication in the Journal of the American Chemical Society, Joseph Tuccinardi and John Wood from Baylor University, USA, introduced a novel strategy to efficiently synthesize the core scaffold of aleutianamine. Their study involved a vinylogous Mukaiyama-Michael reaction cascade using two previously unknown partners. This approach adds to existing methods by providing a new entry point for synthesizing this complex scaffold.
However, once the key fragment was secured, the real challenges began, as late-stage modifications proved to be difficult. As an outsider, it is impressive to see how the team overcame the obstacles encountered during this synthesis, ultimately leading to the successful synthesis of the desired natural product.
Read more: Convergent Total Synthesis of Aleutianamine JACS doi: 10.1021/jacs.4c12946