Synthesis of Quaternary Centres using Lithium Enolates in a Ru-catalysed Allylic substitution
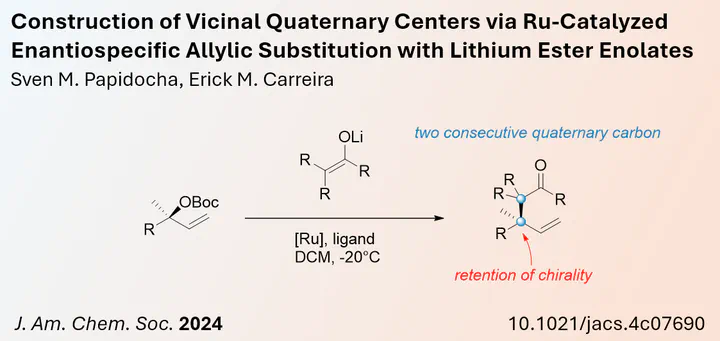
The construction of quaternary centres represents a significant challenge when employing C-C bond formation strategies. Therefore, the development of new methods, in particular stereoselective methods for the formation of such sterically crowded motifs, is warranted. In a published article in the Journal of the American Chemical Society, Erick Carreira and Sven Papidocha from ETH Zürich reported a new synthetic access to such molecules using a combination of lithium enolates and Ru-catalysed allylic substitution. Both of these substrates are under-explored. Lithium enolates are often overlooked because their high reactivity and instability can lead to side reactions, despite their ease of access.
In their study, they have been able to show that the enolate substitution is under complete stereoretention. The authors propose a double inversion process in which the Ru complex is first added and then replaced by the enolate. By using alpha-disubstituted enolates, two vicinal quaternary centres can be set. This new method may contribute to the existing repertoire of allylic substitutions, and further studies may demonstrate its potential.
Read more: Construction of Vicinal Quaternary Centers via Ru-Catalyzed Enantiospecific Allylic Substitution with Lithium Ester Enolates doi:10.1021/jacs.4c07690