Exploring Anti-Bredt Olefins as Reactive Intermediates
Every student in chemistry will encounter the Bredt rule at least once in their studies. In brief, it states that cyclic compounds cannot have double bonds at bridgehead positions due to angle strain and steric hindrance, rendering them unstable or unable to exist. Although this rule has been challenged several times since its formulation in the early 20th century, it remains widely accepted.
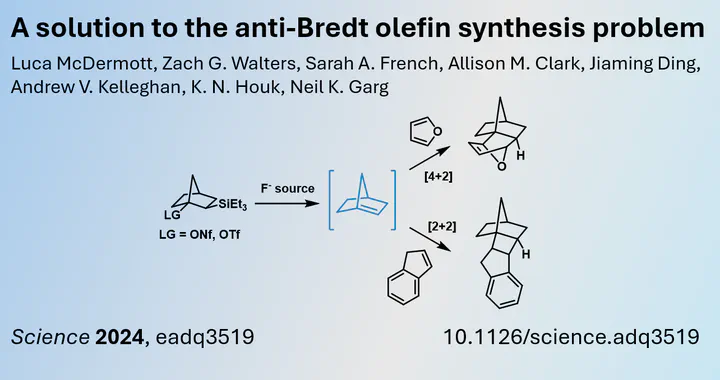
Recently, a research team led by Neil K. Garg from the University of California (USA) published findings on access to anti-Bredt type olefins as intermediates. These intermediates can be obtained by treating a silylated bicyclo compound with fluoride, which triggers elimination at the bridgehead due to a leaving group (e.g. OTf, ONf, Br). This concept was inspired by pioneering work from Chan and Massuda in 1977 (10.1021/ja00445a042). The olefin can then participate in [2+2] or [4+2] cycloaddition reactions (among others), providing indirect proof of the existence of the anti-Bredt olefin. It is important to note that these olefins cannot be isolated and exist only as transient intermediates.
Read more: A solution to the anti-Bredt olefin synthesis problem doi:10.1126/science.adq3519