Deracemisation lead to enriched alpha Amino Aldehydes
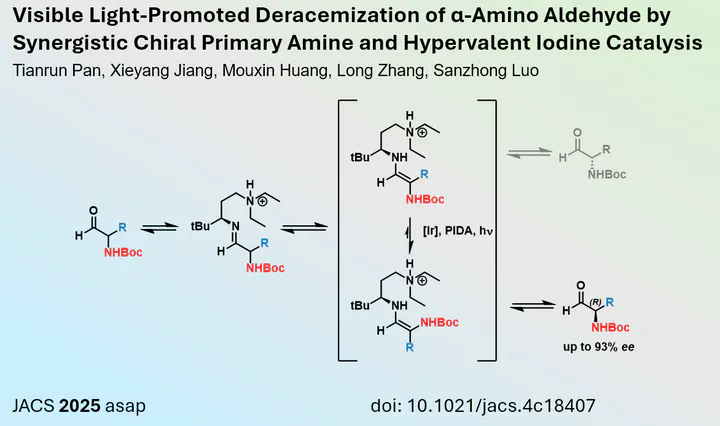
Aldehydes serve as valuable building blocks in chemical synthesis. They can be readily obtained, for example, through the oxidation of alcohols, and their electrophilic nature underpins many carbon-carbon coupling reactions. However, the use of alpha-chiral aldehydes often leads to racemisation during storage or the reaction itself, rendering them less useful. Consequently, methods for the deracemisation of alpha-substituted aldehydes are warranted, as these would provide access to freshly prepared, highly enantioenriched molecules that can be directly transformed into more stable analogues.
In a recent study published in the Journal of the American Chemical Society (JACS), Luo and colleagues from Tsinghua University in Beijing, China, described a novel method for synthesising enantioenriched alpha-amino aldehydes in situ. Their work utilised a chiral amine, which forms an imine with the aldehyde. Following enamine tautomerisation, the (E)/(Z) ratio is favoured towards the (E)-enamine under the influence of iridium photocatalysis, promoted by PIDA. This process ultimately leads to the accumulation of a single enantiomer.
This study is significant as it highlights the potential to deracemise a highly sensitive alpha aldehyde stereocentre, which can subsequently be stabilised through analogue formation (e.g., the Wittig reaction). From a practical standpoint, further studies will determine whether this concept can be applied in synthetic routines. Firstly, the system is highly sensitive; temperature must be meticulously controlled, and the reaction must be conducted in an oxygen-free environment. Furthermore, photocatalysis setups are still relatively rare in many academic laboratories. Although some examples achieve enantiomeric excess exceeding 90%, the typical scale is 0.1 mmol, with the ee of this reaction ranging between 80% and 90%. This may be insufficient for advanced total synthesis campaigns, as the outcomes of scale-up are uncertain, and the separation of enantiomers on a larger scale is less feasible, requiring chiral preparative HPLC.
Read more: Visible Light-Promoted Deracemization of α-Amino Aldehyde by Synergistic Chiral Primary Amine and Hypervalent Iodine Catalysis JACS doi: 10.1021/jacs.4c18407