Macrocycles can lock the Conformation of Drug Candidates and enhance their Activity
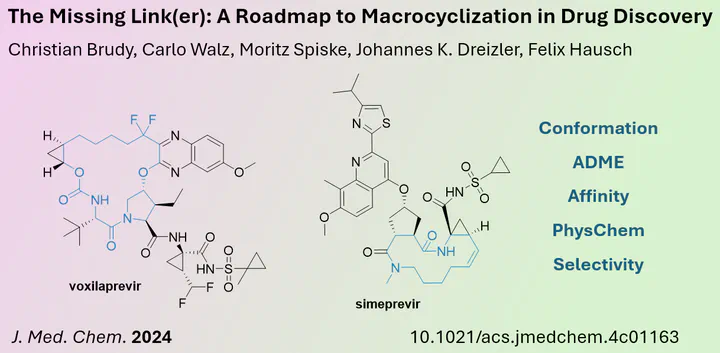
In medicinal chemistry, the conformation of small molecules is critical for enzyme recognition. The process of optimising the structure to fit into the enzyme pocket has been accelerated in recent years by the increasing availability of enzyme/substrate crystallography data and advances in in silico calculations.
In a perspective published in the Journal of Medicinal Chemistry, the research group of Felix Hausch (Technical University of Darmstadt) discusses the concept of using macrocycles to improve and fix the conformation of small molecules. In addition to enhancing biological activity, the authors explore how macrocycles can improve selectivity, ADME properties and target affinity, using recent examples.
In addition, the perspective outlines various linking strategies and explains their role in the macrocycle approach. The authors advocate the use of the macrocycle strategy as they believe that the additional effort required during synthesis can be rewarded with an improved drug candidate.
Read more: The Missing Link(er): A Roadmap to Macrocyclization in Drug Discovery doi:10.1021/acs.jmedchem.4c01163