Selective Nitrosation by Covalent Binder Strategy
Post-translational modifications (PTMs) are changes made to proteins after they have been synthesised. Examples include the addition of a phosphate or acetyl group. These modifications can be carried out by other proteins, and PTMs have recently gained more and more interest as small molecules are now able to induce proximity between proteins, which can then induce such modifications. This strategy is also used in PROTACs, where proteins are ubiquitinated and then degraded. The group I am working with has also demonstrated that O-GlcNAcylation can be induced by OGTACs.
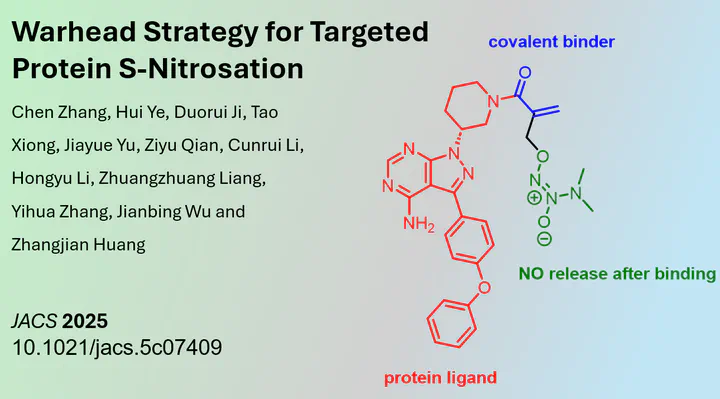
A new member of these molecules has now been published in the Journal of the American Chemical Society. The research group led by Jiangbing Wu and Zhangjian Huang at China Pharmaceutical University in Nanjing described the first small molecules to induce nitrosation. Unlike phosphorylation and acetylation, nitrosation is much less understood. NO itself is a gas, and the addition of NO-generating substances only yielded unselective S-nitrosation.
To overcome this limitation, the researchers presented a bifunctional molecule that, upon covalent binding to its POI (protein of interest — BTK in this case), can release NO in situ. They demonstrated that this resulted in the rapid nitrosation of BTK itself. Although this small molecule is slightly more active than its non-NO parental counterpart, the increase in activity is moderate. The volcano plot indicates that many other proteins are nitrosated. These limitations may be overcome in future publications with improved molecule design. Overall, this study laid the foundations for such research.
Read more: Warhead Strategy for Targeted Protein S‑Nitrosation JACS doi:10.1021/jacs.5c07409