Efficient Synthesis of Chiral ProTides through Copper Catalysis
Nucleoside analogues play an important role in medicinal chemistry and drug development, particularly as antiviral agents. Their efficacy has been demonstrated in the treatment of viral infections such as HIV and hepatitis, primarily by targeting viral polymerases. However, these analogues have to be phosphorylated inside cells in order to be incorporated into viral DNA or RNA, a process that can limit their antiviral efficacy due to slow metabolic activation.
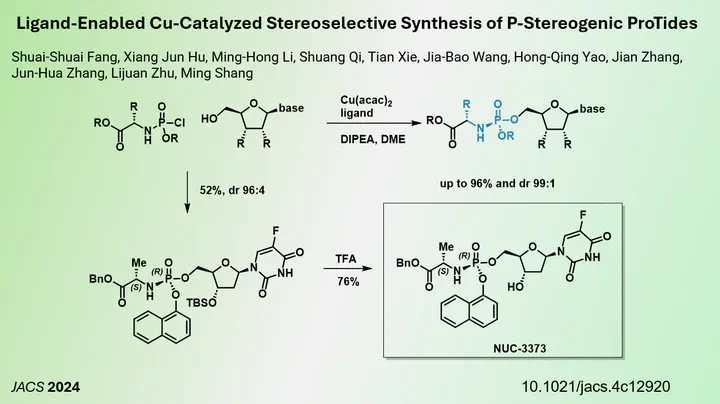
To overcome this challenge, researchers have developed a prodrug strategy using masked monophosphates called ProTide. This approach allows the metabolic release of the active monophosphate within the cell, facilitating its conversion to the bioactive triphosphate form. Despite the promise of this method, the synthesis of ProTide prodrugs can be complicated, mainly because the phosphate group is chiral, requiring stereoselective synthesis or predefined stereopure reagents to avoid lengthy purification processes.
In a recent publication in the Journal of the American Chemical Society (JACS) in November 2024, a collaborative team led by Jun-Hua Zhang, Lijuan Zhu and Ming Shang from Fudan University and Shanghai Jiao Tong University introduced a copper-catalysed method for synthesising these chiral phosphate prodrugs. Using a copper source and a chiral ligand, the researchers demonstrated that racemic chlorophosphates can be effectively converted into their chiral prodrugs.
Their method could be applied to the synthesis of various DNA and RNA nucleoside analogue prodrugs, achieving good yields and stereoselectivity. Through mechanistic studies, the team discovered that while the chiral ligand contributes to stereoselectivity, the presence of copper is critical for achieving maximum selectivity. They conclude, that the ligated copper coordinates to the phosphate which then favours the synthesis one isomer. The study also explores both match and mismatch scenarios as ProTide bear another stereocenter near the phosphate. Overall, this research represents a new tool for the synthesis of ProTide nucleoside analogues, providing an efficient route that can enhance the development of antiviral drugs in future studies.
Read more: Ligand-Enabled Cu-Catalyzed Stereoselective Synthesis of P‑Stereogenic ProTides doi:10.1021/jacs.4c12920