My Reading Tip of the Week: Efficacy of Remdesivir ProDrugs
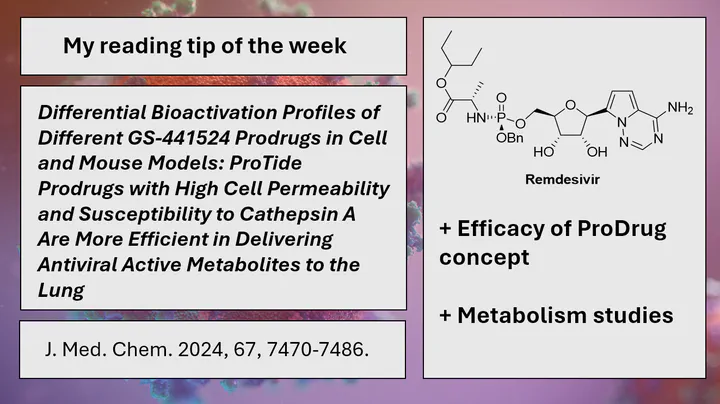
Nucleoside analogues have proven effective as antivirals against many viruses such as HIV. A relatively new nucleoside analogue is remdesivir, which was first introduced against hepatitis and Ebola. Following the outbreak of the SARS-CoV-2 pandemic, it also showed promising antiviral activity in vitro and has also been marketed as a drug against COVID. However, its efficacy in clinical trials has been inconclusive.
One possible reason could be the drug’s metabolism. In order to be active, nucleoside analogues must be triphosphorylated. However, this triphosphorylated form is not a good drug because the high polarity of the phosphate leads to a low ability to cross the cell membrane. Therefore, prodrug concepts are justified and many of them are available. One is the use of a so-called ProTide concept, which is a masked monophosphate. In principle, this prodrug can cross the cell membrane and is then metabolised by host enzymes to the triphosphate.
In a new study published in J. Med. Chem. Hao-Jie Zhu and co-workers from the University of Michigan were able to demonstrate the effect of the ProTide substitution pattern on metabolism to the triphosphate in various cell lines (e.g. Huh-7, Caco-2 and others). The study also demonstrated the efficacy of other prodrugs such as the triester compound. Further in vivo pharmacokinetic studies showed that the protide molecules resulted in higher levels of triphosphate in the lung than the ester prodrug (GS-621763), with higher cell permeability being the key factor.
Read more: Differential Bioactivation Profiles of Different GS-441524 Prodrugs in Cell and Mouse Models: ProTide Prodrugs with High Cell Permeability and Susceptibility to Cathepsin A Are More Efficient in Delivering Antiviral Active Metabolites to the Lung doi:10.1021/acs.jmedchem.4c00234