Pfizer's Second-Generation SARS-CoV-2 Protease Inhibitor
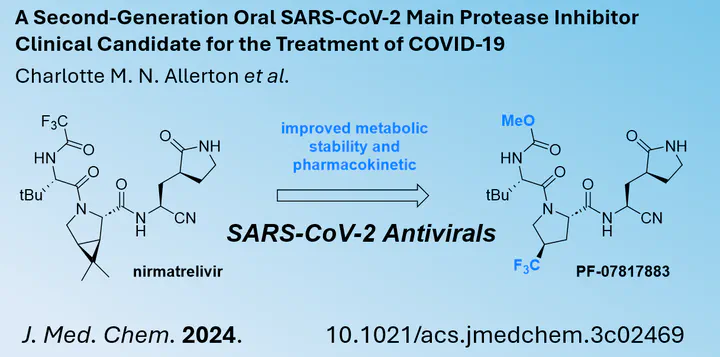
The SARS-CoV-2 pandemic remains a global public health threat. However, in stark contrast to the early days, vaccines and antiviral drugs are now on the market. One of the most effective is Paxlovid, developed by Pfizer, which could minimise the risk of hospitalisation if taken early after the onset of viral infection. Anyone who has already taken this medicine knows that Paxlovid consists of two drugs. One is nirmatrelvir and the other is ritonavir, both taken at the same time. While the former targets the main viral protease (Mpro), which leads to the antiviral activity, the latter is used to slow down the metabolic breakdown of nirmatrelvir. This is done by inhibiting CYP3A4. As this inhibition can lead to side effects or serious interactions with other drugs, there is a need for a main protease inhibitor that is metabolically more stable.
In a study published in the Journal of Medicinal Chemistry, a team of Pfizer researchers has identified a second-generation MPro inhibitor. Its structure is closely related to nirmatrelivir, but improvements have been made to increase metabolic stability without compromising antiviral activity. Having identified a potential candidate, in vivo mouse model studies were able to further demonstrate the antiviral activity by reducing the viral titer in the lungs after 4 days. Importantly, the novel compound can act alone without the need for an additional CYP3A4 inhibitor.
As Pfizer moves this experimental drug forward into clinical trials, this inhibitor may soon be developed into a real market drug. By making small structural changes without affecting the overall conformation of the molecule, it is possible to optimise metabolic stability and improve overall pharmacokinetic properties.
Read more: A Second-Generation Oral SARS-CoV-2 Main Protease Inhibitor Clinical Candidate for the Treatment of COVID-19 doi:10.1021/acs.jmedchem.3c02469