My Reading Tip of the Week: Total Synthesis of Scabrolide A by Radical Cascade
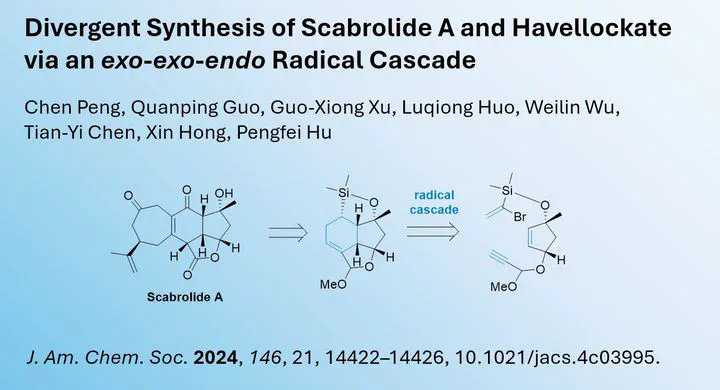
Scabrolide A, a polycyclic marine natural product, has fascinated chemists due to its highly constrained structure. Recently, Pengfei Hu and colleagues from Westlake University in Hangzhou, China, published an innovative strategy for efficiently synthesizing the scaffold of this polycyclic compound.
In their study, which was published in the Journal of the American Chemical Society (JACS), the researchers demonstrated that a radical cascade strategy could be employed to construct the core scaffold. After initiating the radical reaction by AIBN, they successfully formed the central 6-membered cyclohexane ring through an exo-exo-endo triple radical addition cascade with full control over diastereoselectivity.
The final functionalization steps allowed the synthesis of scabrolide A in just nine steps from the central intermediate.
Read more: Divergent Synthesis of Scabrolide A and Havellockate via an exo-exo-endo Radical Cascade doi:10.1021/jacs.4c03995