Ring Closing Alkyne Metathesis allows unified Synthesis of Strasseriolides A to D
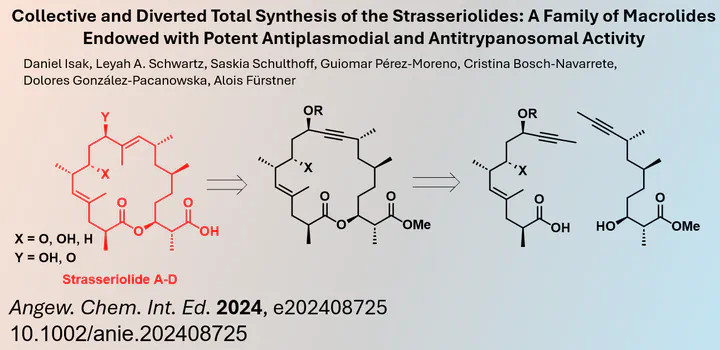
Stasseriolides are a group of natural products classified as polyketides. Polyketides share the common feature of being constructed from C2 units with a repeating pattern of 1,3-diols and/or unsaturated polyenes. In more complex natural products, these units cyclize to form macrocycles and many have shown promising biological activity. Consequently, the synthesis of stasseriolide analogues is an active area of interest for organic chemists, given the limited natural abundance of these compounds.
Strategies for the synthesis of macrocyclic polyketides often involve the iterative construction of linear intermediates, which are then cyclized using common esterification methods such as Yamaguchi or Shiina esterification. Alternatively, in some cases it may be advantageous to close the macrocycle at a different position. This allows a more divergent approach with a shorter number of synthetic steps. For example, I myself used a ring-closing metathesis as the key cyclization step in the synthesis of archazolid F.
Similarly, Alois Fürstner and his team at the Max Planck Institute für Kohlenforschung recently published in the journal Angewandte Chemie the synthesis of stasseriolides A to D using a ring-closing alkyne metathesis reaction. This relatively new synthetic approach not only enabled the preparation of four members of the stasseriolide natural product family, but also allowed the synthesis of a small series of analogues.
This research is a must read for anyone interested in total synthesis, particularly polyketide synthesis, as it employs many useful synthetic routines for the construction of typical polyketide motifs.
Read more: Collective and Diverted Total Synthesis of the Strasseriolides: A Family of Macrolides Endowed with Potent Antiplasmodial and Antitrypanosomal Activity doi:10.1002/anie.202408725