Synthesis of Drug Libraries using Sulfamide Linker Strategy
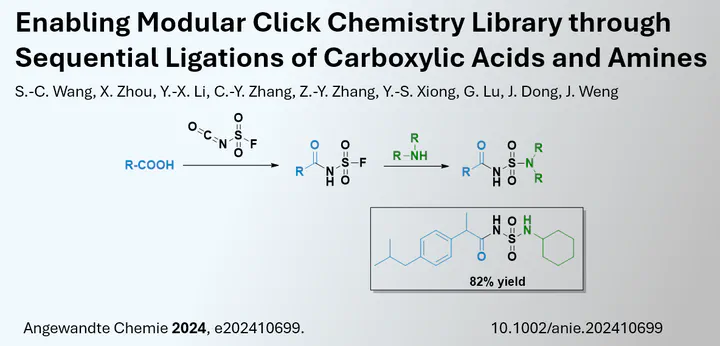
The synthesis of drug libraries is essential in drug discovery projects. However, the synthesis of molecules can often be lengthy and inefficient, resulting in only a few compounds even after large synthesis projects. This is particularly true for academic research facilities and small companies. New strategies are therefore needed. Especially with the emergence of bifunctional molecules (e.g. Protacs), one strategy may be to couple two fragments to form a new drug. In a paper published in the journal Angewandte Chemie, Jiang Weng and co-workers from Sun Yat-Sen University (Guangzhou, China) describe the coupling of the fragments by a sulfamide coupling strategy.
The researchers point out that this new strategy is free from any coupling reagent. By exploiting the inherent coupling properties of a fluorosulfuryl isocyanate fragment, two functional groups (acid and amine) can be coupled via a sulfamide. Of course, acid and amines can be coupled by various other methods, but in this report it was possible to do so on a 96-well plate without any additives. Despite this achievement, improvements still need to be made as a solvent change is still required in this two-step process. In addition, the products need to be purified before testing, as the direct use of the compounds in the 96-well plate for testing was not described.
Read more: Enabling Modular Click Chemistry Library through Sequential Ligations of Carboxylic Acids and Amines doi:10.1002/anie.202410699