Biocatalytic diversification of thionucleoside analogues
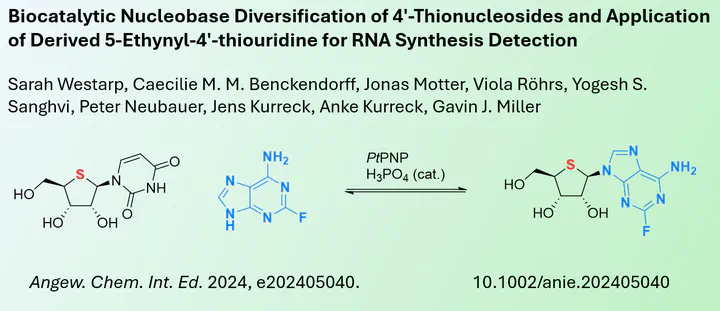
Nucleoside analogues can be used to treat many diseases, and their use against viruses and cancer is an important part of modern medicine. These drugs can be structurally very diverse and one group of them are the thionucleosides. In thionucleosides, the ribose oxygen is replaced by a sulphur atom, which slightly alters the conformation of the ribose and stabilises the drug against hydrolysis. However, the synthesis of these artificial thionucleosides is less efficient than that of their ribose counterparts, so far fewer thionucleoside analogues are known.
In a new report published in Angewandte Chemie, researchers from the Keele University and Technical University of Berlin describe the successful biocatalytic approach for the synthesis of thionucleosides. The concept of enzymes catalysing transglycosylation meaning nucleobase exchange is well known, but not for thionucleosides. Gavin J. Miller’s team showed that although the conversion is lower than with ribose, it is still efficient enough to replace the original uracil base with various purine and pyrimidine nucleobases. The researchers were also able to apply this strategy to the synthesis of 5-ethynyl-4’-thiouridine, which can be used for labelling by click chemistry in cellular medium.
Read more: Biocatalytic Nucleobase Diversification of 4’-Thionucleosides and Application of Derived 5-Ethynyl-4’-thiouridine for RNA Synthesis Detection doi:10.1002/anie.202405040