Breaking the aromaticity of thiophene using a [4+3] cycloaddition
The concept of aromaticity is central to organic chemistry. The formation of aromatic systems is a driving force for many reactions, as their formation is energetically favorable. Taking this into account, the dearomatization of these systems is often accompanied by low yields, reversibility, or the need for extreme conditions.
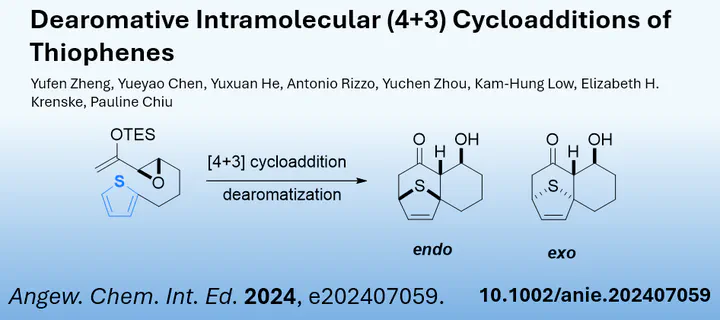
One class of such aromates are thiophenes. Previously their ability to undergo dearomative [4+3]-cycloadditions has been less explored. Now Pauline Chiu and her team at the Hong Kong University reported such a dearomatization reaction of thiophene. In this intramolecular [4+3] cycloaddition, not only is the aromaticity of the thiophene ring broken, but this process also occurs at very low temperatures.
The authors were able to demonstrate the scope of this reaction, which yields complex tricyclic ring systems. Furthermore, in-depth DFT studies could explain the high reactivity, where the intramolecular and irreversible nature of the transformation acts as the driving force. Finally, using a desulfurization strategy, the researchers were able to deliver hydrobenzo[7]annulene frameworks.
Read more: Dearomative Intramolecular (4+3)-Cycloadditions of Thiophenes 10.1002/anie.202407059